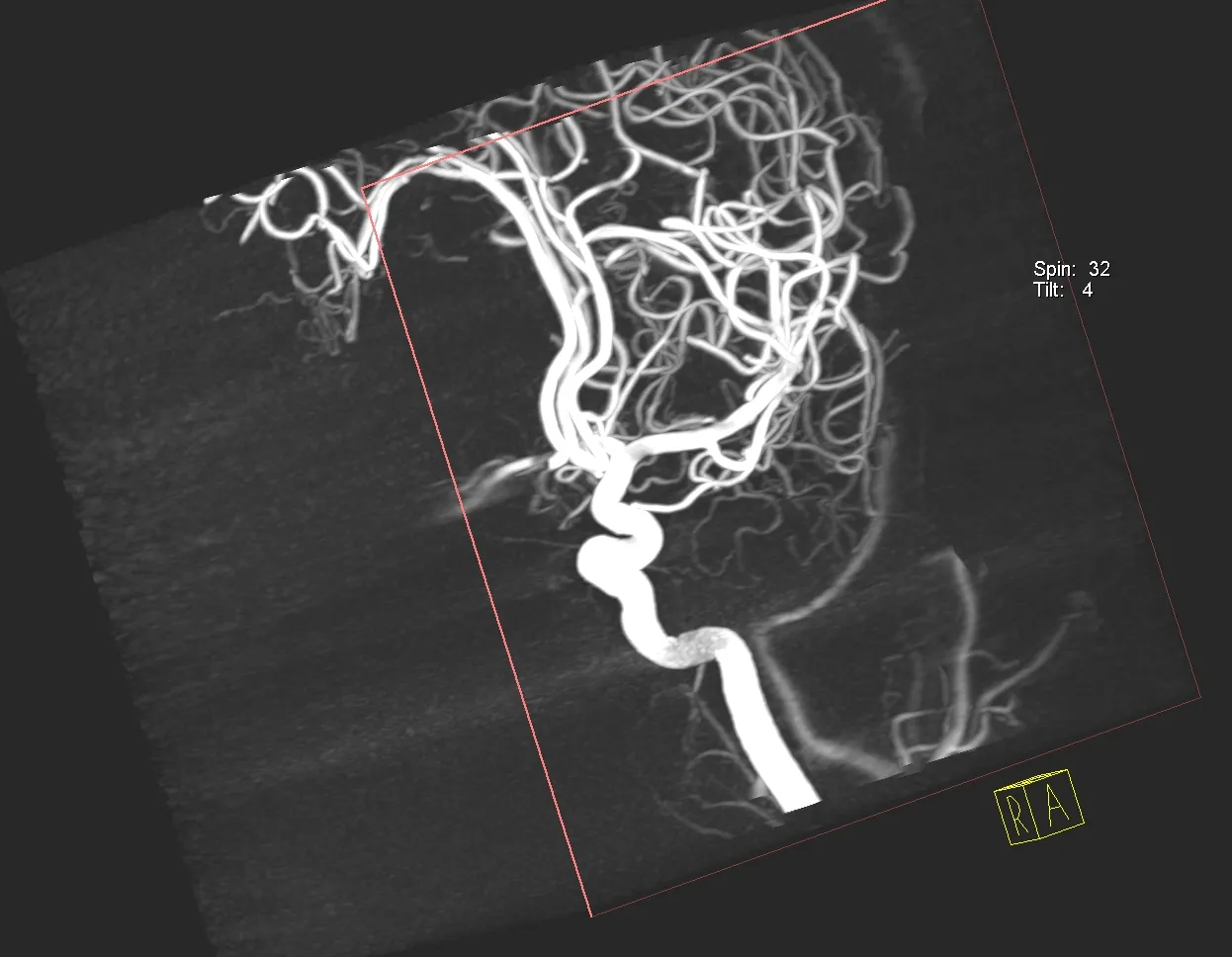
Interventional Stroke Research
Abdulnasser Alhajeri, MD, is a neurointerventional radiologist and a clinical associate professor of radiology. His research interests lie in the area of intervention for stroke treatment. He has been involved in several clinical trials for stroke treatment particularly in the evaluation of various devices used in stroke treatment.
These trials included:
- WEAVE Trial – Wingspan Stent System Post Market Surveillance Study Sponsor - Stryker Neurovascular: This trial was a prospective single arm registry study spanning 50 US and international sites. The primary objective of this trial was to evaluate the rate of stroke and death within 72 hours in patients treated with the Wingspan Stent System with Gateway PTA Balloon Catheter.
- Trevo Registry Trial Sponsor - Stryker Neurovascular: The Trevo retriever is a device to remove thrombus from the neurovasculature in the setting of acute ischemic stroke. This trial was a prospective, open label, consecutive enrollment, multi-center, international trial. Enrollment was to include up to 2,000 patients in up to 100 hospitals around the world. The goal of collecting study information on the TREVO retriever was to increase knowledge of the safety of the device and to possibly help in treatment of future patients with intracranial atherosclerotic disease.

- Safety and Effectiveness of the Treatment of Wide Neck, Saccular IntracraniaL Aneurysms with the Neuroform Atlas Stent System, “ATLAS” Sponsor - Stryker Neurovascular: The purpose of this trial was to evaluate safety and effectiveness of the Neuroform AtlasTM Stent System in the endovascular treatment of wide neck, intracranial, and saccular aneurysms. It is a prospective, multicenter, single arm, open-label, interventional study. The pivotal trial includes a maximum of 30 clinical sites. The primary objectives of the study are to evaluate the effectiveness of the Neuroform AtlasTM Stent system as an adjunct to embolic coiling for the endovascular treatment of wide neck, intracranial, saccular aneurysms by comparing complete aneurysm occlusion rates achieved with stent- assisted coiling to historical occlusion rates achieved with coiling alone.
- Stratis Registry Trial Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke Registry Sponsor - Covidien: This was a prospective, multicenter, non-randomized, observational registry of acute ischemic stroke patients who underwent treatment with the use of a Covidien market-released neurothrombectomy device (as the initial device used to remove the thrombus). Expected enrollment was to include up to 1000 patient in up to 60 sites. Objectives of the study were to assess clinical outcomes and factors that may affect these outcomes.
In all of these studies, Dr. Alhajeri was assisted by his co-investigator, Justin Fraser, MD