The Curry Lab
Overview of Research Interests in the Curry Laboratory
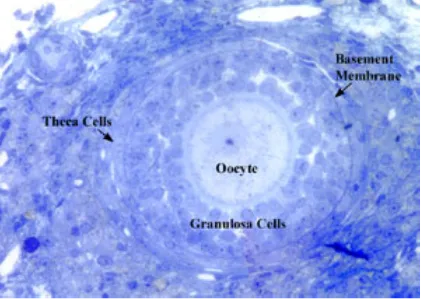
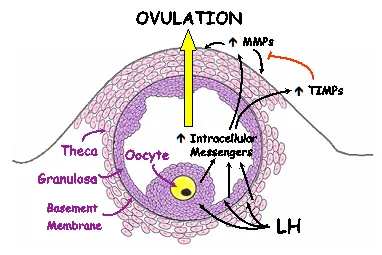
Our laboratory focuses on ovarian function. Specifically, we are interested in the basic question of how the egg or oocyte is released from the ovarian follicle during the process of ovulation. The ovarian follicle is composed of the oocyte and supporting cumulus cells surrounded by the granulosa cells and theca cells that make steroids (Figure 3). In response to midcycle LH surge, the follicular cells secrete paracrine factors that set in motion the process of oocyte release.
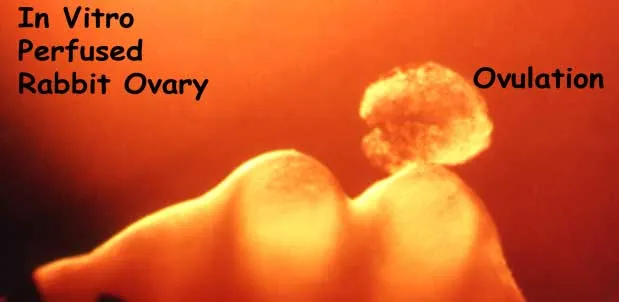
The micrograph in Figure 1 illustrates ovulation in the rabbit. The preovulatory follicle on the left has not ruptured and the oocyte and surrounding cumulus cells have been extruded.
We have a longstanding interest in the involvement of family of proteolytic enzymes, known as matrix metalloproteinases (MMPs), with the process of ovulation. The MMPs are responsible for connective tissue remodeling throughout the body. We have observed that the midcycle surge of LH, which sets in motion the ovulatory process, results in an increase in the expression of mRNA and activity for certain members of the MMP family prior to ovulation. These MMPs may act to break down the follicle wall.
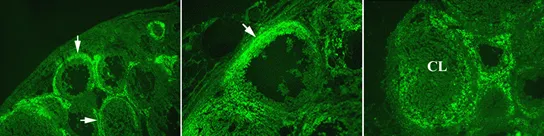
For example, there is an increase in gelatinase activity around the growing follicle and at the apex of the ovulatory follicle as indicated by the green fluorescence (Figure 2).
After ovulation, gelatinase activity increases at the base of the postovulatory follicle as it transforms into the corpus luteum (Figure 2). When MMP activity is inhibited by exogenous enzyme inhibitors, ovulation is inhibited. These observations, that LH induces MMPs and that the inhibition of metalloproteinase action blocks oocyte release, suggest a crucial role for the MMPs in the process of breaking down the follicle wall and oocyte release.
Of particular interest is the finding that enzymatic activity in the extracellular space appears to be regulated by enzyme inhibitors present in the ovary and that LH stimulates an increase in both the MMPs and these inhibitors, known as tissue inhibitors of metalloproteinases or TIMPs (Figure 3). We hypothesize that LH acts through various intracellular messengers to stimulate the expression and activity of the MMPs. These proteinases in turn act to degrade the extracellular matrix at the apex or top of the follicle. Degradation of the follicle wall results in a thinning of the apex, rupture of the follicle wall and release of the oocyte. The TIMPs act to control the extent of proteinase activity.
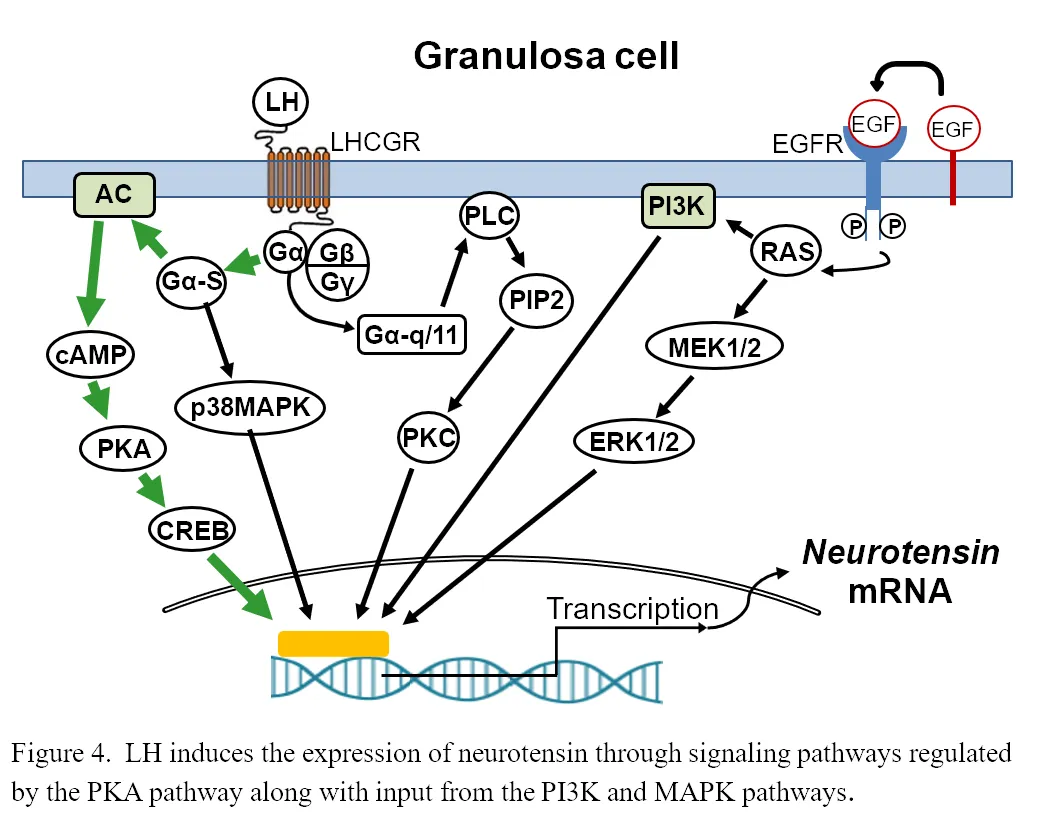
Recently, we have collected preovulatory follicles from women across the periovulatory period and are identifying potential key regulators in the ovulatory process. During this process of discovering new ovulatory mediators, we have found that neurotensin (NTS), a small neurotransmitter, was massively induced during the early and late ovulatory stage in human granulosa cells (15,000 fold) and theca cells (700 fold). This induction occurs through the signaling pathways regulated by the protein kinase A (PKA) pathway along with input from the PI3K and MAPK pathways (Figure 4).
Blocking NTS action results in hemorrhagic luteinized unruptured follicles suggesting that NTS plays role in the vascular remodeling associated with ovulation. We have blocked NTS action with a silencing RNA approach and are in the process of identifying downstream targets of NTS with an RNA sequencing approach with the overall goal of characterizing the actions of neurotensin in the ovulatory process.
Aging and Ovarian Function
Another area of research emphasis in our laboratory is the exploration of aging and ovarian function. These studies are aimed at determining the interaction between the loss of reproductive function with aging, the cohort of ovarian follicles, and changes in ovarian genes of interest during follicular growth. These studies were initiated as part of a consortium project with other investigators at the University to explore the ovarian and neuroendocrine axis during reproductive aging in the rat. An example of some of the work performed exploring the follicular populations is seen here. Shown is a section of a rat ovary cut and stained by Tammie Tyler, one of our previous undergraduate BIOL 395 students. The photomicrograph clearly illustrates the different follicular compartments such as the oocyte, the granulosa layer, and the thecal layer in a secondary follicle. This information is being used to evaluate the follicular populations during the aging process in the rat.