Second Heart Field–Derived Cells Contribute to Angiotensin II–Mediated Ascending Aortopathies
View Recent publication on Circulation
Authors
Hisashi Sawada, Yuriko Katsumata, Hideyuki Higashi, Chen Zhang, Yanming Li, Stephanie Morgan, Lang H. Lee, Sasha A. Singh, Jeff Z. Chen, Michael K. Franklin, Jessica J. Moorleghen, Deborah A. Howatt, Debra L. Rateri, Ying H. Shen, Scott A. LeMaire, Masanori Aikawa, Mark W. Majesky, Hong S. Lu and Alan Daugherty
Abstract
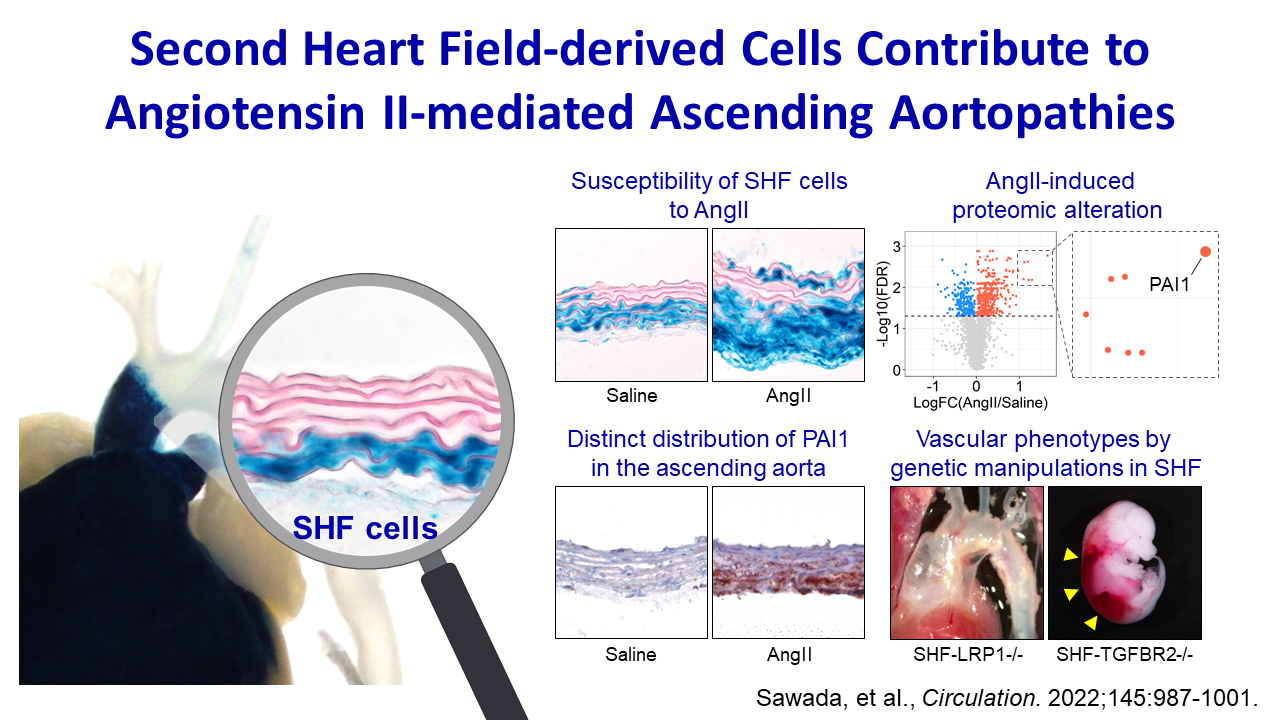
Background:
The ascending aorta is a common location for aneurysm and dissection. This aortic region is populated by a mosaic of medial and adventitial cells that are embryonically derived from either the second heart field (SHF) or the cardiac neural crest. SHF-derived cells populate areas that coincide with the spatial specificity of thoracic aortopathies. The purpose of this study was to determine whether and how SHF-derived cells contribute to ascending aortopathies.
Methods:
Ascending aortic pathologies were examined in patients with sporadic thoracic aortopathies and angiotensin II (AngII)–infused mice. Ascending aortas without overt pathology from AngII-infused mice were subjected to mass spectrometry–assisted proteomics and molecular features of SHF-derived cells were determined by single-cell transcriptomic analyses. Genetic deletion of either Lrp1 (low-density lipoprotein receptor–related protein 1) or Tgfbr2 (transforming growth factor–β receptor type 2) in SHF-derived cells was conducted to examine the effect of SHF-derived cells on vascular integrity.
Results:
Pathologies in human ascending aortic aneurysmal tissues were predominant in outer medial layers and adventitia. This gradient was mimicked in mouse aortas after AngII infusion that was coincident with the distribution of SHF-derived cells. Proteomics indicated that brief AngII infusion before overt pathology occurred evoked downregulation of smooth muscle cell proteins and differential expression of extracellular matrix proteins, including several LRP1 ligands. LRP1 deletion in SHF-derived cells augmented AngII-induced ascending aortic aneurysm and rupture. Single-cell transcriptomic analysis revealed that brief AngII infusion decreased Lrp1 and Tgfbr2 mRNA abundance in SHF-derived cells and induced a unique fibroblast population with low abundance of Tgfbr2 mRNA. SHF-specific Tgfbr2 deletion led to embryonic lethality at E12.5 with dilatation of the outflow tract and retroperitoneal hemorrhage. Integration of proteomic and single-cell transcriptomics results identified PAI1 (plasminogen activator inhibitor 1) as the most increased protein in SHF-derived smooth muscle cells and fibroblasts during AngII infusion. Immunostaining revealed a transmural gradient of PAI1 in both ascending aortas of AngII-infused mice and human ascending aneurysmal aortas that mimicked the gradient of medial and adventitial pathologies.
Conclusions:
SHF-derived cells exert a critical role in maintaining vascular integrity through LRP1 and transforming growth factor–β signaling associated with increases of aortic PAI1.