Functional Neuroplasticity in the Nucleus Tractus Solitarius and Increased Risk of Sudden Death in Mice with Acquired Temporal Lobe Epilepsy.
Click here to be directed to PubMed page
Functional Neuroplasticity in the Nucleus Tractus Solitarius and Increased Risk of Sudden Death in Mice with Acquired Temporal Lobe Epilepsy.
Author information
- 1
- Department of Physiology, College of Medicine, University of Kentucky, Lexington, KY 40536.
- 2
- Epilepsy Center, University of Kentucky, Lexington, KY 40536.
Abstract
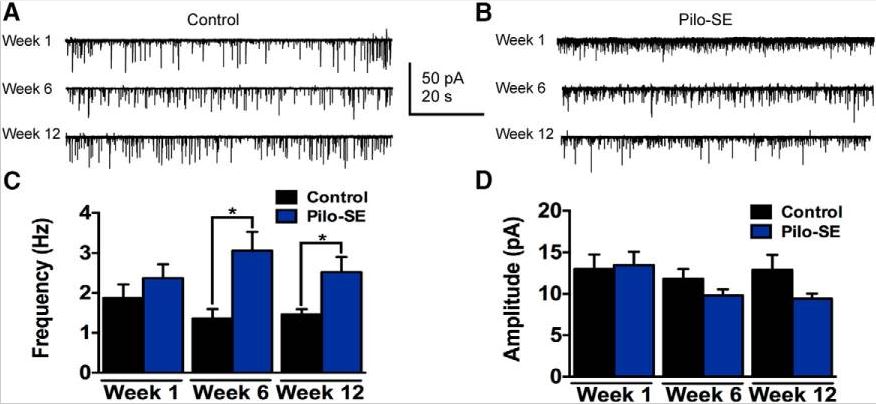
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death in individuals with refractory acquired epilepsy. Cardiorespiratory failure is the most likely cause in most cases, and central autonomic dysfunction has been implicated as a contributing factor to SUDEP. Neurons of the nucleus tractus solitarius (NTS) in the brainstem vagal complex receive and integrate vagally mediated information regarding cardiorespiratory and other autonomic functions, and GABAergic inhibitory NTS neurons play an essential role in modulating autonomic output. We assessed the activity of GABAergic NTS neurons as a function of epilepsy development in the pilocarpine-induced status epilepticus (SE) model of temporal lobe epilepsy (TLE). Compared with age-matched controls, mice that survived SE had significantly lower survival rates by 150 d post-SE. GABAergic NTS neurons from mice that survived SE displayed a glutamate-dependent increase in spontaneous action potential firing rate by 12 wks post-SE. Increased spontaneous EPSC frequency was also detected, but vagal afferent synaptic release properties were unaltered, suggesting that an increase in glutamate release from central neurons developed in the NTS after SE. Our results indicate that long-term changes in glutamate release and activity of GABAergic neurons emerge in the NTS in association with epileptogenesis. These changes might contribute to increased risk of cardiorespiratory dysfunction and sudden death in this model of TLE. KEYWORDS:
Autonomic; EPSC; GABA; brainstem; epilepsy; vagus
- PMID:
- 29085908
- PMCID:
- PMC5661358
- DOI:
- 10.1523/ENEURO.0319-17.2017