Project Leaders
W. Brad Hubbard, PhD
Investigating and Treating Metabolic Deficits in the Neurovascular Unit Following Mild Traumatic Brain Injury
W. Brad Hubbard, PhD, assistant professor, Spinal Cord & Brain Injury Research Center and UK Department of Physiology (May 2023 - present)
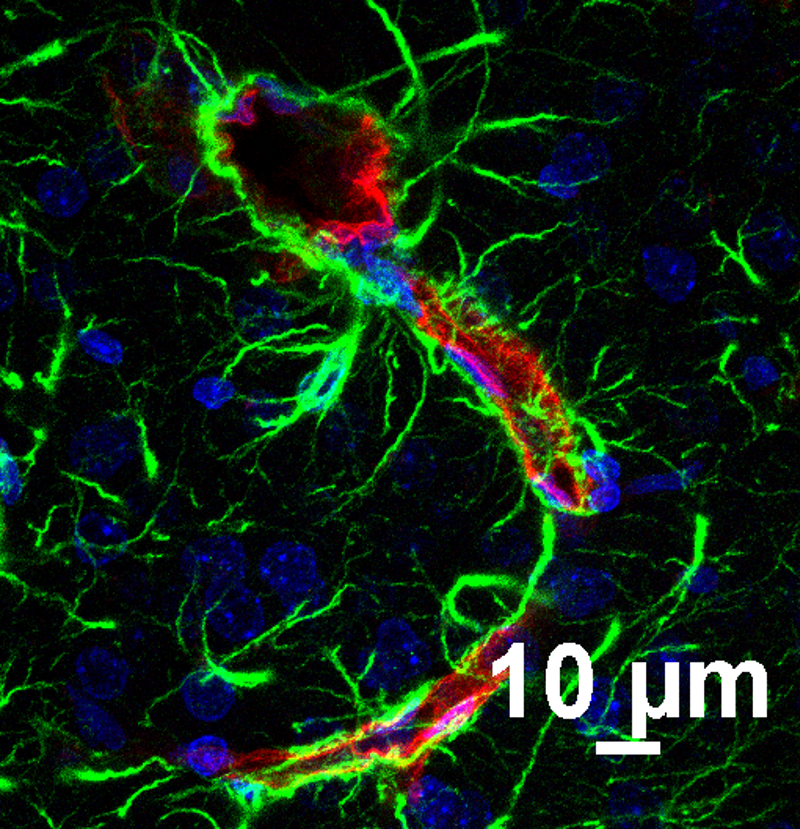
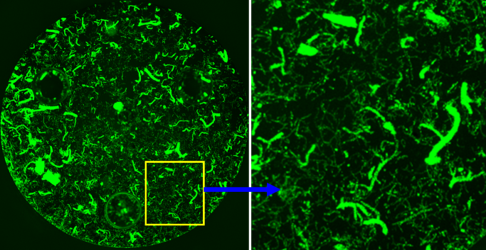
Traumatic brain injuries (TBIs) are a major societal and public health concern with over 1.7 million TBIs, ~80% of which mild, are reported each year in the US. TBI is also a major risk factor for dementias and Alzheimer’s Disease. Mild TBI is characterized by dysfunction of the neurovascular unit (NVU), initiated by breakdown of the blood-brain barrier and followed by synaptic dyshomeostasis. Mitochondrial dysfunction is apparent in the NVU after mild TBI, accompanied by metabolic imbalance and oxidative damage. This project on mild TBI will examine the metabolic deficits of distinct components of the NVU, including neuronal synapse and brain capillaries, using the strengths of CNS Metabolism COBRE Cores. This project also examines the therapeutic effect at restoring NAD+ related alterations in metabolism and functional outcomes to identify promising candidates to treat mild TBI.
Mentors: Bjoern Bauer, Adam Bachstetter
Tritia Yamasaki, MD, PhD
Identifying Metabolomic Markers in Conversion to Cognitive Impairment in Parkinson's Disease
Tritia Yamasaki, MD, PhD, associate professor, UK Department of Neurology
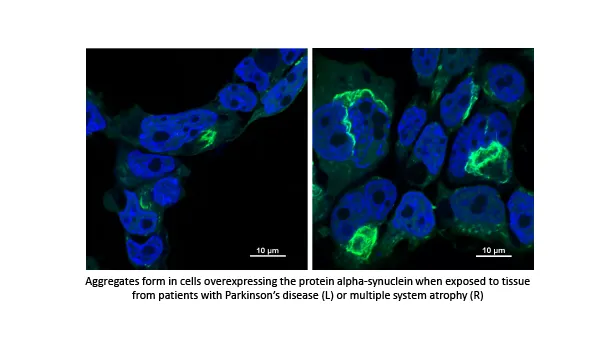
Parkinson's disease is a debilitating and progressive neurodegenerative disease. When combined with cognitive impairment, morbidity, mortality and caregiver burden increase exponentially. Cognitive impairment can occur in 50-80% of people with Parkinson's disease, but the timeline to development and the rapidity of progression is currently unpredictable. Prior work in our lab has identified specific pathways that are dysregulated in Parkinson's disease. Our project aims to assess metabolomic profiles in biofluids of the Parkinson's patients to help identify those that may be likely to convert to cognitive impairment. This would help guide choice of therapeutics and stratify groups for potential interventions.
Mentors: John Slevin, Frederick Schmitt
Amelia K. Pinto, PhD
Amelia Pinto, PhD, assistant professor, UK Department of Microbiology, Immunology. and Molecular Genetics
Investigating the role of cellular metabolism on infection and disease.
Over 100 million Americans have Metabolic Syndrome (MetS). MetS is characterized by disruptions in cellular metabolism, including altered glucose utilization, lipid dysregulation, and chronic inflammation. These metabolic changes can impair cellular energy production and immune function, creating an environment that influences susceptibility to infections. My research project focuses on understanding how MetS associate disruptions in cellular metabolism in the brain leads to a dysregulation of viral replication and enhanced disease. Specifically, we investigate the metabolic pathways that are hijacked or altered by neurotropic viruses to enhance their replication and survival within neural tissues. By examining the interplay between viral infection and key metabolic processes such as glucose metabolism, lipid biosynthesis, and mitochondrial function, our work aims to identify how these pathways contribute to viral replication efficiency and disease progression. This research not only sheds light on the fundamental mechanisms of host-virus interactions in the brain but also provides insights into potential metabolic targets for therapeutic intervention to mitigate viral infections and associated neurological complications.
Graduated
Maj-Linda Selencia, PhD
Sustained eIF5A Hypusination at the Core of Brain Metabolic Dysfunction in TDP-43 Proteinopathies
Co-morbidities such as those contributing to metabolic diseases (e.g., diabetes) are known risk factors for dementia. Studies support that regional decline in glucose metabolic rate in the brain and reduced blood flow denotes impaired energy metabolism as one of the earliest and most consistent features of Alzheimer’s Disease. However, the causal role of TDP-43 pathology to AD pathogenesis and metabolic status remains elusive. Our laboratory has identified hypusination of eukaryotic translation initiation factor 5A (eIF5A), as a novel pathway upstream of synaptic plasticity impairments, cognitive decline, and neurodegeneration—cascades linked to the irreversible stages of dementia. We reported earlier on that reduction of hypusination via pharmacological inhibition of deoxyhypusine synthase enzyme (DHS), significantly reduced TDP-43 phosphorylation and accumulation in the cytoplasm and stress granules in cellular stress model. We demonstrated that reducing sustained hypusination rescues cerebral metabolic dysfunction and TDP-43 pathogenesis in mice. The ongoing studies in our laboratory will elucidate how eIF5AHyp regulates brain metabolism and neuronal mitochondrial bioenergetics. These studies will establish the eIF5AHyp pathway and the singly hypusine moiety as a therapeutic target for TDP-43 pathogenesis in ADRD and LATE dementia.
Maj-Linda Selencia, PhD
Sustained eIF5A Hypusination at the Core of Brain Metabolic Dysfunction in TDP-43 Proteinopathies
Co-morbidities such as those contributing to metabolic diseases (e.g., diabetes) are known risk factors for dementia. Studies support that regional decline in glucose metabolic rate in the brain and reduced blood flow denotes impaired energy metabolism as one of the earliest and most consistent features of Alzheimer’s Disease. However, the causal role of TDP-43 pathology to AD pathogenesis and metabolic status remains elusive. Our laboratory has identified hypusination of eukaryotic translation initiation factor 5A (eIF5A), as a novel pathway upstream of synaptic plasticity impairments, cognitive decline, and neurodegeneration—cascades linked to the irreversible stages of dementia. We reported earlier on that reduction of hypusination via pharmacological inhibition of deoxyhypusine synthase enzyme (DHS), significantly reduced TDP-43 phosphorylation and accumulation in the cytoplasm and stress granules in cellular stress model. We demonstrated that reducing sustained hypusination rescues cerebral metabolic dysfunction and TDP-43 pathogenesis in mice. The ongoing studies in our laboratory will elucidate how eIF5AHyp regulates brain metabolism and neuronal mitochondrial bioenergetics. These studies will establish the eIF5AHyp pathway and the singly hypusine moiety as a therapeutic target for TDP-43 pathogenesis in ADRD and LATE dementia.