Research Focus
Understanding the chronic SCI environment
While much has been done to understand the development of acute and sub-acute SCI pathology, much less is known about how the chronic SCI environment interacts with damaged and spared neuronal tissue. Many of the major non-resolving perturbations at the lesion are not well described. Interrogating how chronic SCI lesions interact with damaged and spared neuronal tissue to mediate regenerative failure and/or ongoing neuronal functions is vital to advance effective treatments for chronic SCI.
Determine the role of non-resolving neuroinflammation on repair and functions in chronic SCI
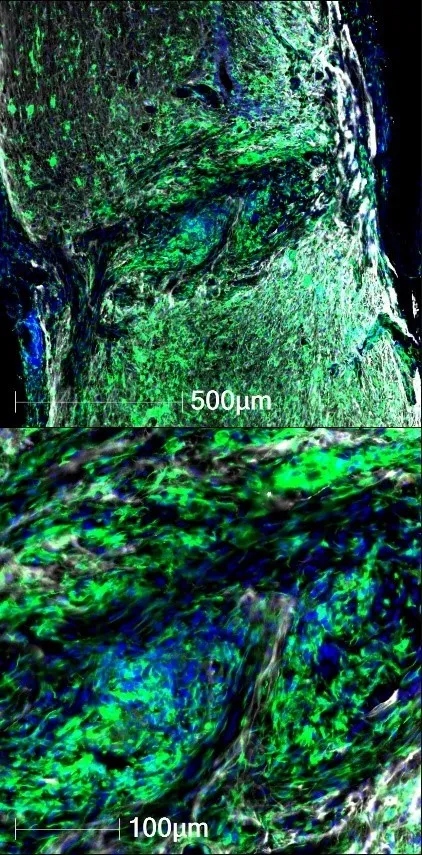
Upon looking at a chronic SCI lesion, sustained inflammation is one of “the biggest elephants in the room”. What sustained inflammation does to neuronal tissue and its role in mediating or inhibiting repair and regeneration is not well described. We have recently observed that depleting inflammation from the lesion core can augment the growth of specific axon fiber types within the lesion, suggesting that chronic inflammation plays an inhibitory role in repair. Better understanding the growth-inhibitory nature of non-resolving inflammation may lead to strategic targets to begin repairing the chronically injured spinal cord.
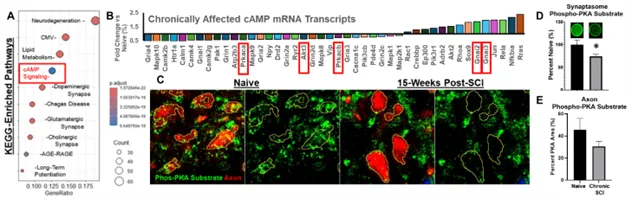
Understanding the role of sustained disruptions to the neural phosphoproteome in chronic SCI.
One major regulator of not just axon growth, but also neuronal excitability and functions, is the balance of the neural phosphoproteome. The expression and activity of several intracellular phosphatases remains high in neurons surrounding the lesion, as well as the expression and activation of receptor-ligand interactions that suppress vital cell-signaling kinases such as PKA. Using transcriptional analysis, phosphoproteomics, and immunohistochemistry, we have identified a sustained disruption to both the PKA and AKT cell-signaling pathways in neurons; two pathways vital for axon regeneration and signal propagation. We aim to better understand how disruptions to these cell-signaling pathways affect the potential for repair as well as ongoing cellular functions during chronic stages of SCI.
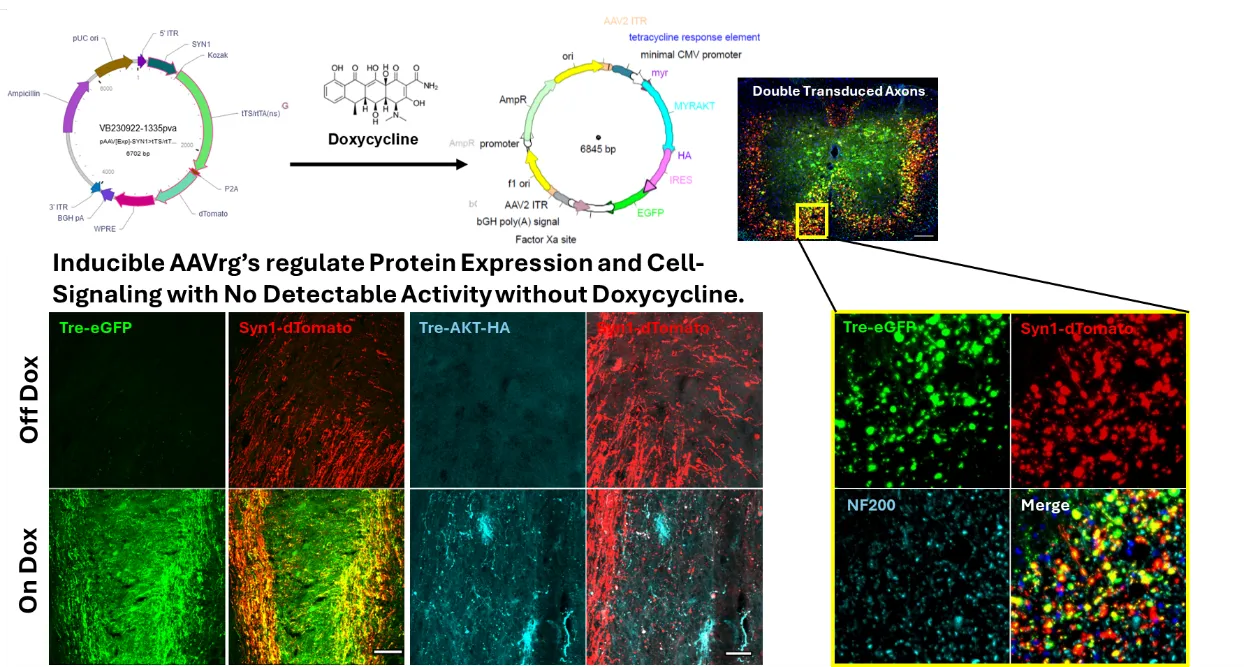
Development of gene therapies to treat chronic SCI.
Axons damaged from SCI do not spontaneously regenerate. The more we learn about mechanisms that support regeneration within the spinal cord, the more we appreciate that growth and regeneration is largely regulated by both genetic responses and cytosolic cell-signaling pathways. Neither an appropriate genetic response, nor an appropriate orchestration of cell-signaling activity, is initiated within axons after SCI. For regeneration to be successful, it is likely that we will need to control both transcriptional activity, as well as the activity of strategic cell-signaling pathways. To date, gene therapies are arising as the most specific, safe, and controllable approach to manipulate gene expression and cell-signaling activity in vivo. Our prior work has demonstrated that gene-therapies can limit the growth effects to just the spinal-projecting axons of interest and are working on advancing mechanisms to provide temporal control of transgene expression and cell-signaling activity.
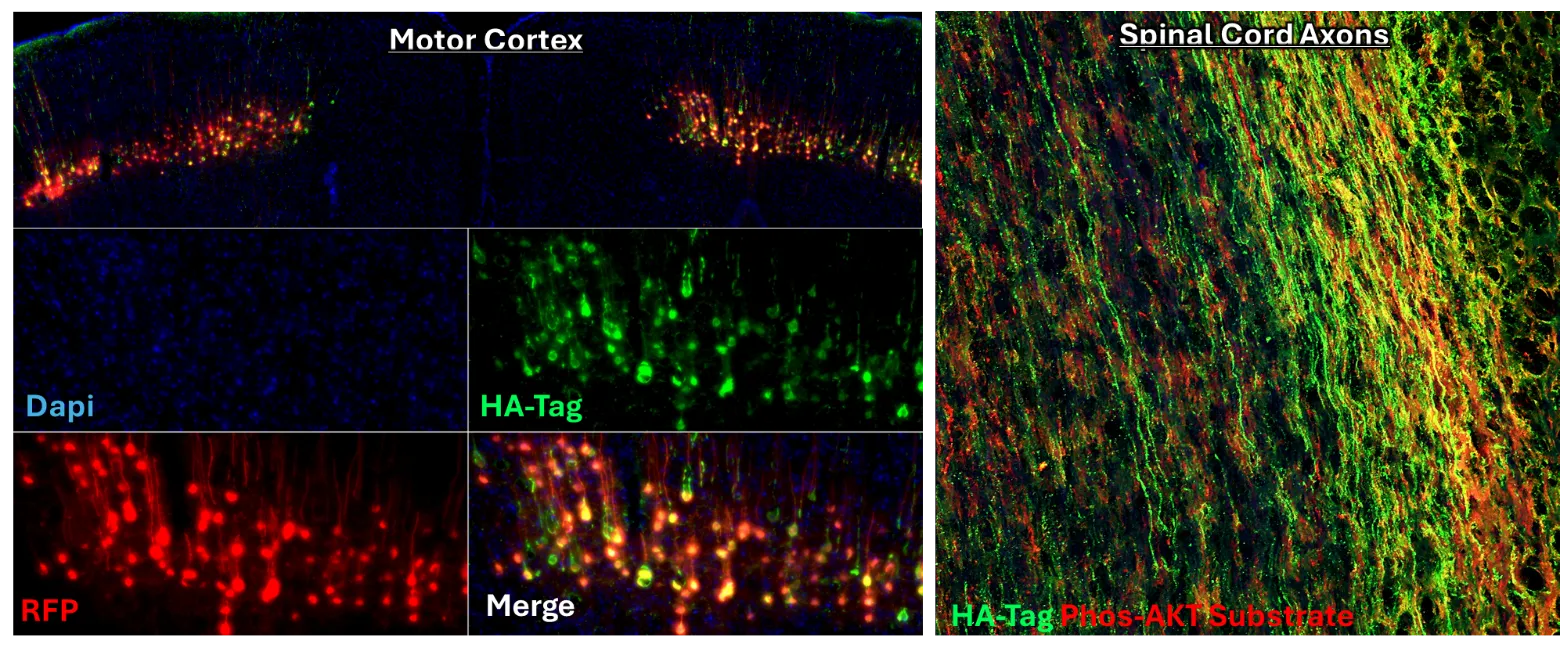
Identifying and therapeutically targeting intracellular mechanisms that either restrict, or promote, the regeneration of damaged axons.
By leveraging advancements in gene-delivery technology, we have demonstrated that targeting spinal-projecting axons using a novel viral vector (AAVrg) can improve functions through both growth-dependent, and independent means. Our approach enables the interrogation of the role of several different novel targets in both axon growth and function that can be leveraged for therapeutic gain. As an ongoing research focus, our lab develops and refines novel approaches to engage regenerative pathways in neurons using gene-therapies tailored to the chronic SCI pathology.
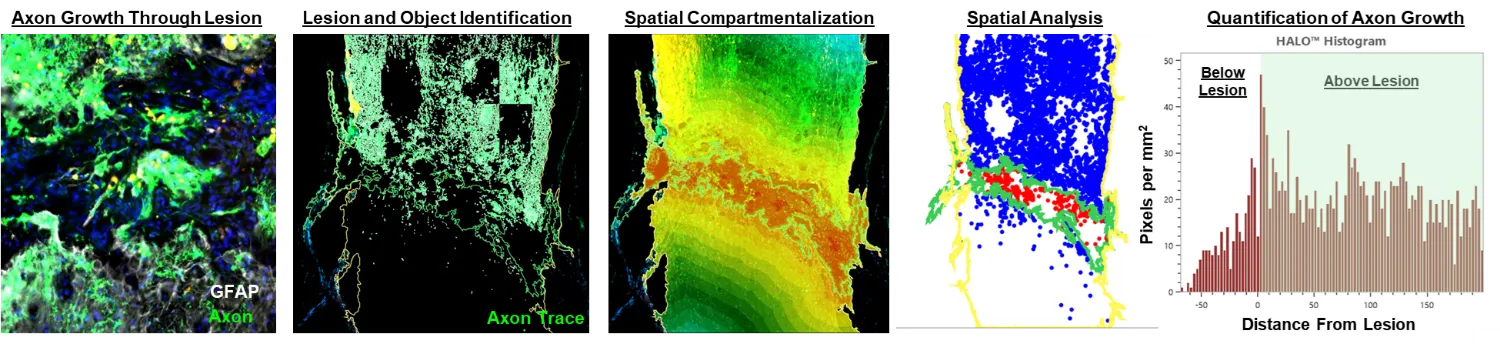
Refine the safety and delivery approaches for applying gene therapies after SCI.
Our long-term goal is to translate a clinically viable treatment approach that exhibits efficacy to restore functions in chronic SCI. To that end, we increasingly appreciate that no two spinal cord injuries are identical, with sex, age, and injury dynamics affecting treating outcomes, sometimes in opposing directions. Identifying target populations who will benefit from our gene-therapy approaches remains vital for successful translation. We are manipulating several targets that have restored functions in chronic SCI to define the delivery and demographic variables that predict efficacious outcomes. This includes the creation of novel genetic vectors that are inducible in a time- and cell-specific manner to optimize the needed duration to maintain trans-gene expression.
Promote a growth-permissive matrix to support axon regeneration in chronic SCI.
In both humans and rats alike, the chronically injured spinal cord is hallmarked by large fluid filled cavities. For axon regeneration to be successful, some kind of supportive matrix is required for growth. There is a need for combinatorial approaches to not just coerce the growth of axons, but to provide a compatible extracellular matrix to support regeneration. We have begun to tackle this regenerative requirement by understanding the preferences of different axon sub-types for different types of extracellular ligands. By understanding the need for tissue-specific antigens to support regeneration, we can genetically engineer stem cells to provide a more permissive extracellular matrix to support repair.
Determine the cell-cell interactions that support growth of both sensory and motor axons.
Our prior work has supported the idea that the chronic lesioned environment does not act like central nervous system (CNS) tissue. The lesion center is filled with peripheral cells including immune cells and fibroblasts that provide antigens otherwise foreign to CNS axons. Correspondingly, several peripheral nervous system axon subtypes can grow permissively within the lesion, while very few CNS axons grow within the lesion. Our lab had begun interrogating how and why some peripheral axons maintain growth potential into chronic SCI lesions, while CNS-derived axons largely do not. We aim to identify necessary antigens that can be provided through exogenous means, or to force the expression of specific receptors on CNS axons, to enable regeneration through the lesion.
Genetically-engineer stem cells to support regeneration of damaged motor axons.
Filling the cystic lesion cavity with a growth-permissive substrate is necessary to support axon growth. Several stem cell sources have already been safely applied to human SCI conditions which establishes a precedent that cell-grafts may be an ideal combinatorial approach to regenerate the damaged spinal cord. Our lab seeks to identify key approaches that can safely and effectively enhance the regeneration of damaged axons by utilizing genetic engineering approaches to modify stem cells ex vivo prior to transplanting into chronic models of SCI.