Recent Faculty Publications: "Unfolding the Story of Proteoglycan Accumulation in Thoracic Aortic Aneurysm and Dissection."
Unfolding the Story of Proteoglycan Accumulation in Thoracic Aortic Aneurysm and Dissection.
Author information
- 1
- From the Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston (Y.H.S., S.A.L.).
- 2
- Department of Cardiovascular Surgery, Texas Heart Institute, Houston (Y.H.S., S.A.L.).
- 3
- Saha Cardiovascular Research Center (H.S.L., A.D.), University of Kentucky, Lexington.
- 4
- Department of Physiology (H.S.L., A.D.), University of Kentucky, Lexington.
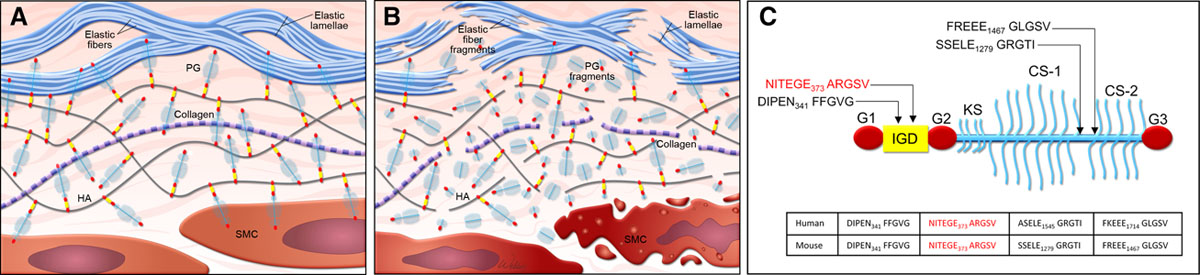
Proteoglycans are important components of the extracellular matrix in vascular tissue and exert critical roles in maintaining aortic structure and function.1 In the normal vasculature (Figure [A]), proteoglycans such as versican interact with hyaluronan to form large aggregates that enhance water retention and create reversible, compressive structures that are necessary for smoothing out pressure waves and abrogating the deformation of blood vessels.2–5 Normal proteoglycan aggregates also pressurize the intralamellar spaces and promote the connection between smooth muscle cells and elastic laminae in the arterial wall, thereby facilitating mechanosensing.5 In addition, proteoglycans maintain aortic hemostasis by regulating elastic fiber assembly6,7 and smooth muscle cell proliferation.3,8 However, the abnormal accumulation of proteoglycans (Figure [B]) can be destructive to aortic structure and function. Recent studies using computational simulations have suggested that an abnormal increase in proteoglycan aggregate mass in the aorta can increase intralamellar swelling pressure, disrupt cell-matrix interactions, and delaminate the microstructure of the aortic wall, leading to compromised aortic mechanosensing and structural integrity.5,9,10 Thus, an imbalance between proteoglycan production and turnover may disturb the integrity of aortic structure and function.1,5,9,10
In normal vascular tissue, the abundance of proteoglycans is low, but it is increased significantly in vascular disease.1 Abnormal proteoglycan accumulation has been detected frequently in the degenerative aortic media of thoracic aortic aneurysm and dissection (TAAD) tissues, as described in mucoid extracellular matrix accumulation11 and the outdated concept of cystic medial necrosis.12 The acidic glycosaminoglycans in proteoglycans give alcian blue staining its characteristic bluish-green color. Accumulation of glycosaminoglycans is observed often in areas with severe elastic fiber fragmentation and smooth muscle cell loss. Recently, using liquid chromatography–tandem mass spectrometry analysis, Cikach et al13 and Yin et al14 have identified different proteoglycans and glycopeptides in ascending aortic tissues from patients with TAAD, including those with Marfan syndrome. Large aggregates of aggrecan and versican were detected in ascending aortas from patients with TAAD and mice with Marfan syndrome,13,14 particularly in dissected and ruptured aortas.13 These findings have suggested that the accumulation of aggregated proteoglycans, particularly aggrecan and versican, in the aortic media contributes to aortic degeneration in TAAD. Thus, it is critically important to understand the regulation of proteoglycan production, degradation, and turnover and the interaction between proteoglycan metabolism and other components of the aortic wall.
Proteoglycans are cleaved by matrix metalloproteases and ADAMTSs (the presence of a disintegrin-like and metalloprotease domain with thrombospondin-type motifs). In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Dupuis et al15 describe the importance of ADAMTS5-mediated aggrecan cleavage for normal aortic wall development. The authors showed that Adamts5-deficient (Adamts5−/−) mice displayed anomalies in the ascending aorta, such as increased aortic thickness, aortic stenosis, altered outflow tract rotation and aortic geometry, smooth muscle cell loss, and blood cells in the aortic wall. These aortic abnormalities were associated with aggrecan accumulation. The authors further identified the site of ADAMTS5-mediated aggrecan cleavage that was important for aggrecan turnover and aortic development. Using antibodies that recognized neo-epitopes of aggrecan fragments generated by ADAMTS-mediated cleavage (Figure [C]), they observed that cleaved aggrecan fragments with FFGVG, SSELE, FREEE, and GLGS neo-epitopes accumulated in aortas of Adamts5−/− mice. In contrast, they observed a reduced abundance of cleaved aggrecan fragments with the TEGE neo-epitope. These findings indicate that ADAMTS5 may be involved in aggrecan cleavage at the TEGE373↓374ALGSV site. Furthermore, the authors demonstrated that mice carrying a noncleavable mutation at this site (TEGE373↓374NVYS) displayed mild aortic abnormalities, suggesting that cleavage at this site may be important for aggrecan turnover and normal aortic wall development. These findings are in line with those of several recent studies that support an emerging role of ADAMTS5 as a key enzyme involved in proteoglycan turnover and aortic protection in mice.16,17 Indeed, mice lacking the catalytic domain of ADAMTS5 (Adamts5Δcat) showed aggrecan accumulation and thoracic aortic dilatation,16 as well as exacerbated ascending aortic enlargement in mice administered angiotensin II.17 Further analyses revealed that the accumulation of an ADAMTS-specific versican cleavage product (versikine) was decreased, and that the abundance of versican was increased in Adamts5Δcat mice.17 These findings suggest that the ADAMTS5-mediated cleavage and turnover of aggrecan and versican are critical for aortic morphogenesis during development and the maintenance of aortic integrity throughout adulthood. Others have reported the increased production of proteoglycans (particularly aggrecan and versican) in the aortic wall of TAAD tissues.13 Together, these findings have raised the possibility that aggrecan and versican accumulation in ascending TAAD may result from increased production and reduced turnover of these proteoglycans.13–17
The elegant study by Dupuis et al15 leads to several additional questions. First, it is not clear whether induction of proteoglycan production results from aortic inflammation or the adaptive response to aortic injury. Further work is needed to determine the factors and pathways that stimulate proteoglycan production and to characterize the dynamic roles of proteoglycan metabolism in aortic inflammation, injury, repair, and remodeling. Second, the comprehensive regulation of proteoglycan cleavage is not well understood. Proteoglycans are important for the homeostasis of aortic structure and function. Unwanted proteoglycan cleavage not only causes the disruption of normal proteoglycan function but also generates cleaved products that can further promote an inflammatory response. In contrast, insufficient proteoglycan cleavage and turnover cause abnormal proteoglycan accumulation. Therefore, the precise control of proteoglycan cleavage is critical. It is not clear whether site-specific cleavage is specifically responsible for degrading functional proteoglycans or clearing dysfunctional proteoglycan fragments. The possibility also remains that cleavage at the same site, through more complex regulation, has diverse effects on proteoglycan processing, degradation, and clearance. Finally, it is likely that different ADAMTS family members such as ADAMTS1,18 ADAMTS5,15–17 and ADAMTS419 are differentially involved in proteoglycan metabolism. Their specific proteoglycan substrates and their regulation of the specific cleavage sites on each proteoglycan remain to be determined. The upstream stressors and pathways that regulate ADAMTS expression and activity, as well as subsequent proteoglycan dysfunction and turnover also remain to be identified. With the increasing use of advanced technologies such as proteomics (glycoproteomics and proteoglycanomics) and conditional knockout mice, our understanding of the dynamic regulation of proteoglycan metabolism will continue to unfold.
Acknowledgments
We gratefully acknowledge Nicole Stancel, PhD, ELS(D), of Scientific Publications at the Texas Heart Institute, for editorial support and Scott Weldon, MA, FAMI, from the Division of Cardiothoracic Surgery, Baylor College of Medicine, for creating the illustrations.
Sources of Funding
Our research activities are supported by grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (HL131980, HL143359, HL127111, HL133723, and HL139748) and from the American Heart Association (AHA) Vascular Diseases Strategically Focused Research Networks (SFRN; AHA18SFRN33960114). The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA. Dr LeMaire’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine.
KEYWORDS:
ADAMTS proteases; Editorial; aggrecans; aortic aneurysm; aortic dissection; extracellular matrix; proteoglycans
- PMID:
- 31553667
- PMCID:
- PMC6764585
- [Available on 2020-10-01]
- DOI:
- 10.1161/ATVBAHA.119.313279