"Lysyl Oxidase Inhibition Ablates Sexual Dimorphism of Abdominal Aortic Aneurysm Formation in Mice"
Abdominal aortic aneurysm (AAA) formation involves a complex process of aortic medial destruction through degradation of extracellular matrix proteins, elastin, and collagen. AAA exhibits sexual dimorphism because male sex is a major risk factor of AAA in both mice and humans.1 In mice, testosterone has been implicated as a major contributor to sexual dimorphism of angiotensin II (AngII)–induced AAAs.2 However, the mechanism by which testosterone drives sexual dimorphism of AAA is unknown. During aortic development, lysyl oxidase (LOX) covalently cross-links elastin and collagen to create an insoluble extracellular matrix resistant to proteolytic degradation.3 We hypothesized that testosterone-mediated suppression of LOX activity is a critical contributor to increased susceptibility of male to AAA formation. Ethics and institutional review board approvals were provided by University of Kentucky (Protocol-2011-0907) and the Ehime University (Protocol-1603002), and informed consent was received from all participants.
To test this hypothesis, we first evaluated LOX protein in human male and female, nonaneurysmal and aneurysmal, abdominal aortic sections. LOX protein was significantly more abundant in the aortic media in female nonaneurysmal and aneurysmal aortic sections than in male nonaneurysmal and aneurysmal aortic sections (Figure A). Consistent with observations in humans, abdominal aortas from female C57BL/6J mice showed significantly more LOX protein in the aortic media than from male mice (Figure B). To discern whether this sexual dimorphism was functionally relevant, we examined LOX activity by measuring breakdown of fluorescent-labeled LOX substrate in aortic tissues from male and female mice infused with either saline or AngII (1000 ng·kg–1·min–1) for 7 days. Consistent with LOX protein abundance, LOX activity in female aortic tissue was ≈2.5-fold significantly higher than in male aortic tissue, and AngII infusion had no influence on LOX activity in either sex (Figure C). To explore whether sex hormones regulate aortic LOX activity, 8-week-old male and female mice were subjected to either castration (orchiectomy or ovariectomy) or sham operation. Four weeks after surgery, abdominal aortic LOX activity was measured. Orchiectomy of male mice not only significantly increased LOX activity, in comparison with sham controls, but to a level present in female mice. Ovariectomy had no influence on LOX activity in female mice in comparison with sham controls (Figure D). Furthermore, testosterone replacement by dihydrotestosterone pellet (16 µg/d subcutaneously) administration for 2 weeks significantly suppressed LOX activity in orchiectomized mice similar to sham controls (Figure E). These results suggest that androgens, not estrogens, regulate and suppress aortic LOX activity in mice.
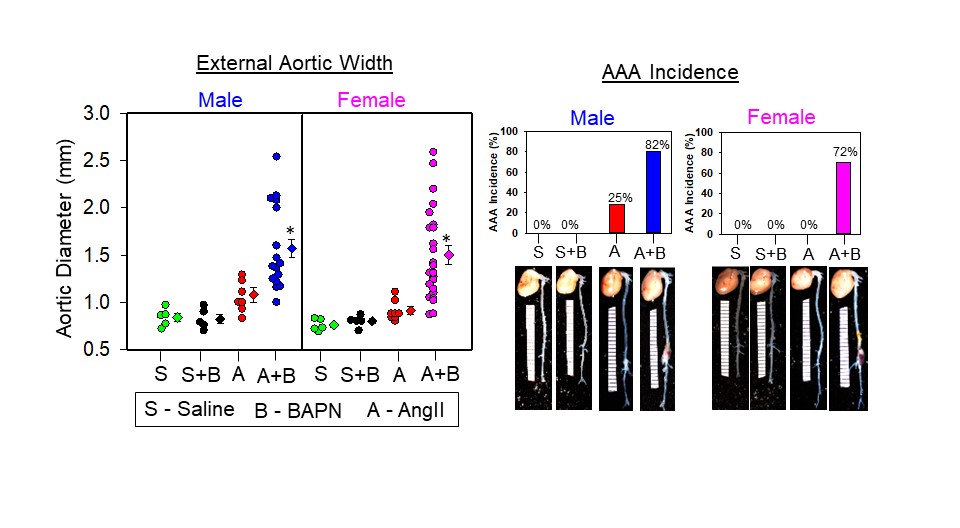
Previous studies have indicated that AngII infusion promotes AAA incidence ≈80% in male hyperlipidemic mice but only ≈10% in male normolipidemic mice. Conversely, AngII infusion caused <10% AAA incidence in both hyperlipidemic and normolipidemic female mice.4 Recently, pharmacological inhibition of LOX by β-aminopropionitrile (BAPN) was shown to promote AngII-induced AAA formation (>80%) in normolipidemic male mice.5 However, the effect of LOX inhibition on sexual dimorphism of AngII-induced AAA in female mice is unknown. To test whether LOX inhibition influences sexual dimorphism of AAAs, male and female mice were infused as follows: (1) saline, (2) saline+BAPN (0.15 g·kg–1·d–1), (3) AngII (1000 ng·kg–1·min–1), and (4) AngII+BAPN. Mice were infused with either saline or AngII through osmotic minipumps for 28 days. BAPN was administered in drinking water (1 mg/mL; 0.15 g·kg–1·d–1) from day 0 to day 14.5 The development of AAA was assessed by (1) ultrasound measurement of aortic luminal dilation, (2) ex vivo measurement of external abdominal aortic width, and (3) AAA incidence defined as 50% increase in luminal dilation in comparison with baseline.
AngII infusion resulted in a 25% (2 of 8) AAA incidence (Figure I–L) with no significant aortic luminal dilation and expansion in comparison with saline/BAPN groups in male but not female mice (Figure F–H). Consistent with previous publications, AngII+BAPN administration increased AAA incidence to 82% (19/23; Figure I and K) with significant luminal dilation and expansion (Figure F–H), along with 30% (7 of 23) aortic rupture in male mice. In female mice, AngII+BAPN administration showed a significant aortic luminal dilation and expansion (Figure F–H) along with a 72% AAA incidence (18 of 25; Figure J and L) and 12% (3 of 25) rupture. These results indicate that pharmacological inhibition of LOX abolishes sexual dimorphism in AngII-induced AAA formation by equally exacerbating aortic dilation Figure. LOX is a novel target for AAA. A, Immunohistochemical staining revealed that LOX protein is more abundant in the media of female human nonaneurysmal and aneurysmal abdominal aortic sections (red indicates positive immunostaining; Abcam, ab31238; n=3, *P<0.05 AAA vs NAA; $P<0.05 female NAA vs male NAA; #P<0.05 female AAA vs male AAA; 2-way ANOVA followed by Holm-Sidak post hoc test). B, LOX protein is more abundant in aortic media of female mouse abdominal aortic sections than in male mouse abdominal aortic sections (n=3, *P<0.05 female vs male; Student t test). C, Aortic LOX activity is lower in male mice infused with either saline or AngII (1000 ng·kg–1·min–1 for 5 days) than in female mice (Abcam, ab112139; n=4, *P<0.05 female vs male; 2-way ANOVA followed by Holm-Sidak post hoc test). D, Orchiectomy (ORCH) not ovariectomy (OVX) increased aortic LOX activity in mice (n=5, *P<0.05 vs male sham; 2-way ANOVA followed by Holm-Sidak post hoc test). E, Testosterone replacement suppressed aortic LOX activity in ORCH mice (n=4–5, *P<0.05 ORCH vs sham; #ORCH+testosterone vs ORCH; 1-way ANOVA followed by Holm-Sidak pairwise multiple comparison test). F and G, LOX inhibition by BAPN (0.15 g·kg–1·d–1) accelerated 28 days infusion of AngII-induced aortic luminal dilation measured by ultrasound (Vivo 2100) in female mice as similar to male mice (n=5–25, *P<0.05 AngII+BAPN vs saline/BAPN/AngII; 2-way repeatedmeasures ANOVA). H though L, LOX inhibition accelerated AngII-induced AAA formation and incidence in female mice as similar to male mice (n=5–25, *P<0.05 AngII+BAPN vs saline/BAPN/AngII; 2-way ANOVA followed by Holm-Sidak post hoc test). M and N, LOX inhibition resulted in loss of medial SMCs (α-SMC actin staining; Abcam, ab5694) along with AngII-induced aortic medial elastin breakage (Verhoeff staining) equivalently in both male and female mice (n=3, *P<0.05 vs male/female saline; 2-way ANOVA followed by Holm-Sidak post hoc test). Scale bars correspond to 50 μm (×200 magnification). O through R, BAPN administration accelerated AngII-induced AAA formation in ORCH mice and testosterone replacement significantly promoted AngII+BAPN–induced AAA rupture in ORCH mice (n=9–10, #P<0.05 ORCH+testosterone vs ORCH; 1-way ANOVA and Fisher exact test). A indicates angiotensin II; AAA, abdominal aortic aneurysm; AngII, angiotensin II; A+B, AngII+BAPN; BAPN, β-aminopropionitrile; LOX, lysyl oxidase; NAA, nonaneurysmal abdominal aorta; S, saline; S+B, saline plus BAPN; SMC, smooth muscle cell; and U/S, ultrasound. Downloaded from http://ahajournals.org by on November 16, 2020 Okuyama et al Lysyl Oxidase in Abdominal Aortic Aneurysms Circulation. 2020;142:1993–1995. DOI: 10.1161/CIRCULATIONAHA.119.044986 November 17, 2020 1995 CORRESPONDENCE and rupture in both male and female mice. Immunohistochemistry and histological analysis revealed that LOX inhibition significantly reduced medial α-smooth muscle cell actin–positive area and promoted AngII-induced medial elastin breakage equivalently in both male and female mice (Figure M and N). BAPN administration promoted AngII-induced AAA formation in orchiectomized mice similar to sham controls (Figure O and P), and testosterone replacement significantly promoted AngII+BAPN–induced AAA rupture (7 of 10) in orchiectomized mice in comparison with orchiectomized controls (0 of 10; Figure Q and R), which strongly demonstrates that maintenance of LOX activity is critical to protect the castrated mice from AAA development.
To our knowledge, this study is the first report to implicate LOX in the sexual dimorphism of AAAs. Stabilization or restoration of LOX may abolish sexual dimorphism and reduce abdominal aortic dilation in males to levels similar to those in females.
ARTICLE INFORMATION
The data, analytic methods, and study materials will be maintained by the corresponding author and made available to other researchers on reasonable request.
Correspondence
Venkateswaran Subramanian, PhD, Saha Cardiovascular Research Center, Department of Physiology, BBSRB - Room 261, University of Kentucky, Lexington, KY 40536-0509. Email venkat.subramanian@uky.edu
Affiliations
Saha Cardiovascular Research Center (M.O., W.J., A.J., J.Z.C., D.A.H., L.Y., V.S.) and Department of Physiology (J.Z.C., V.S.), University of Kentucky, Lexington. Department of Cardiology, Pulmonology, Hypertension, and Nephrology (M.H., J.A.) and Department of Cardiovascular and Thoracic Surgery (T.Y.), Ehime University Graduate School of Medicine, Toon, Japan. Department of Surgery, Division of Vascular Surgery, University of Miami, FL (R.I.V.-P.).
Acknowledgments
We acknowledge the skilled editorial assistance of D. L. Rateri.
Sources of Funding
This study was supported by a Scientist Development Grant (14SDG18740000 to V.S.) from the American Heart Association and by the National Institutes of Health (grants P20GM103527, R01HL130086 to V.S. and R01HL133723).
Disclosures None.
REFERENCES
1. Boese AC, Chang L, Yin KJ, Chen YE, Lee JP, Hamblin MH. Sex differences in abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2018;314:H1137–H1152. doi: 10.1152/ajpheart.00519.2017 2. Henriques TA, Huang J, D’Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866– 3872. doi: 10.1210/en.2003-1615 3. Rodríguez C, Martínez-González J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102 4. Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res. 2012;110:e73–e85. doi: 10.1161/CIRCRESAHA.111.253880 5. Kawai T, Takayanagi T, Forrester SJ, Preston KJ, Obama T, Tsuji T, Kobayashi T, Boyer MJ, Cooper HA, Kwok HF, et al. Vascular ADAM17 (a disintegrin and metalloproteinase domain 17) is required for angiotensin II/β-aminopropionitrile-induced abdominal aortic aneurysm. Hypertension. 2017;70:959–963. doi: 10.1161/HYPERTENSIONAHA.117.09822