FORWARRD
Facilitating Opportunities for Research Workforce Advancement to Retain and Recruit Dynamic Teams
Welcome to FORWARRD
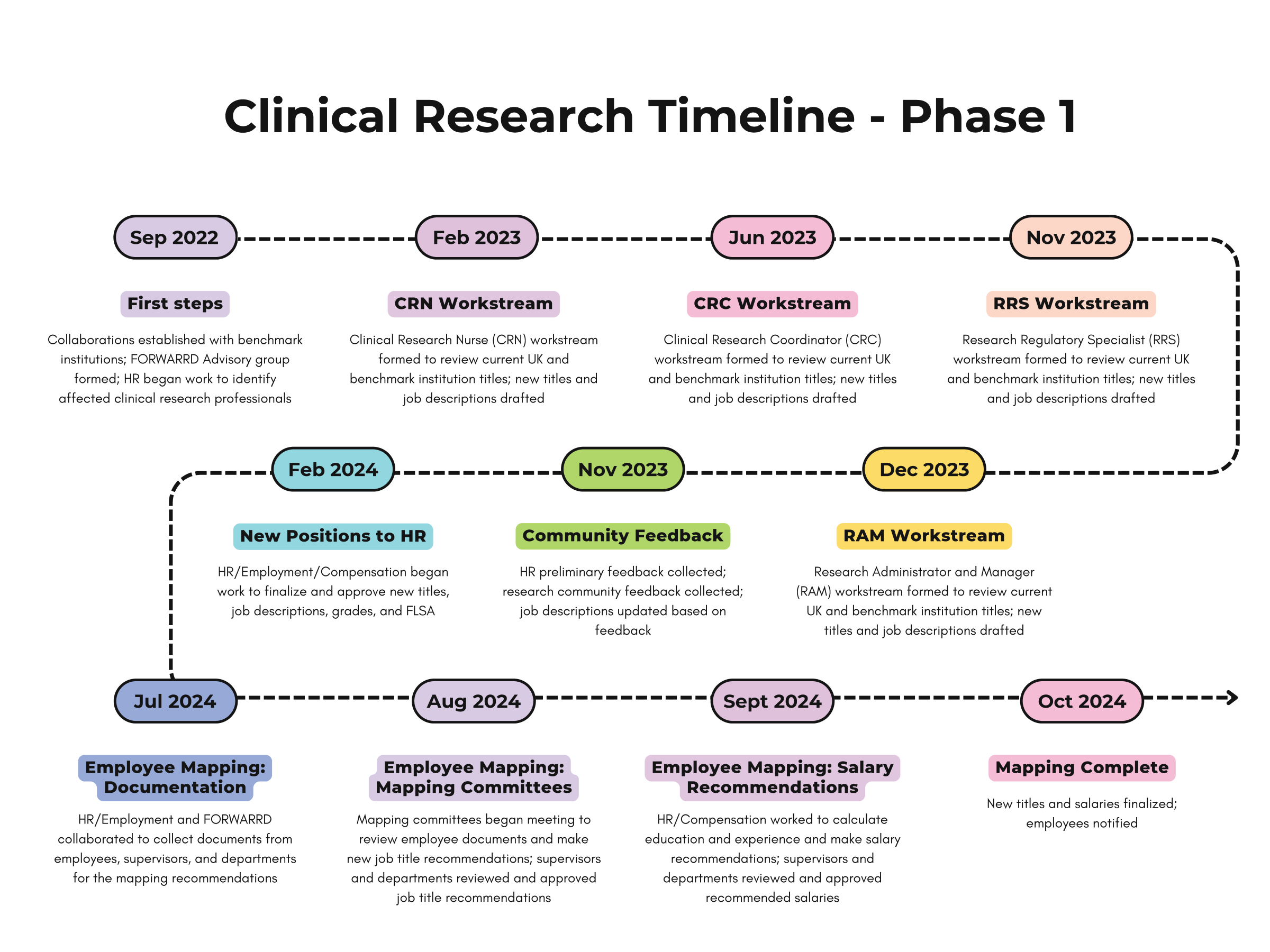
The College of Medicine Office of Research recently launched phase one of its new initiative to elevate research through clinical research professional workforce advancement efforts across the College of Medicine, Markey Cancer Center (MCC), and the Center for Clinical and Translational Science (CCTS).
Facilitating Opportunities for Research Workforce Advancement to Retain and Recruit Dynamic Teams (FORWARRD) is led by Andrea McCubbin, associate dean for research administration. The initiative is based on the Joint Task Force for Clinical Trial Competency’s Framework for clinical research professionals. The Framework’s eight competency domains focus on the knowledge, skills, and attitudes necessary to conduct safe, ethical, and quality research.
Program Goal
Our goal is to create a framework for clinical research jobs in the UK College of Medicine, Markey Cancer Center (MCC), and Center for Clinical and Translational Science (CCTS); from choosing the appropriate job descriptions for new positions to setting professional development goals and providing career advancement opportunities.
Our primary objectives are to:
• Align job titles and descriptions with responsibilities and tasks
• Enhance market competitiveness to attract and retain talented research professionals
• Address the need for competency-based training and career paths

Enhancing the Clinical Research Workforce: A Collaborative Approach with Human Resources
"Revamping job descriptions and creating career ladders in any industry is daunting. Doing it in a traditional AMC clinical research setting where teams have operated in a decentralized, siloed manner may seem impossible. Despite the challenges, endeavors to standardize the CRP positions and career pathways exist using the Joint Task Force for Clinical Trial Competency framework and are well-documented by Duke University (Brouwer et al., 2017; Deeter et al., 2020; Stroo et al., 2020), and further instantiated at the University of Alabama at Birmingham (UAB) in March 2020. More recently, various efforts are underway at other AMCs, several included in this perspective. There are likely other implementations by AMCs in-part, or in-whole that are not known or documented."
Excerpt from "Enhancing the Clinical Research Workforce: A Collaborative Approach with Human Resources." By Denise C Snyder, Heather Gaudaur, Mark Marchant, Laura Viera, Andrea McCubbin, William Verble, Angela Mendell, Christine Gilliam. Published in Frontiers in Pharmacology, section Drugs Outcomes Research and Policies
Joint Task Force (JTF) Competency Framework for Clinical Research Professionals
The JTF Framework has been utilized internationally by academic institutions, corporate entities, and professional societies to improve the quality, accuracy, and safety of clinical research.
The JTF Framework has categorized the following competency domains for clinical research professionals:
- Scientific concepts and research design
- Ethical and participant safety considerations
- Investigational products development and regulation
- Clinical study operations (GCPs)
- Study and site management
- Data management and informatics
- Leadership and professionalism
- Communication and teamwork