Additional Power Point Presentation Available on NIH Public Access Policy
Questions continue to come up related to compliance with the NIH Public Access Policy. We are pleased to provide this additional resource as investigators work through the steps to comply with this policy. This PowerPoint is from a presentation at the June 2013 NIH Regional Conference. To view the presentation, click on the file below.
NIH RPPR Available for Non-SNAP Progress Reports on April 25
NIH Will Open the Research Performance Progress Report (RPPR) for All Type 5 Non-SNAP Progress Reports on April 25, 2014
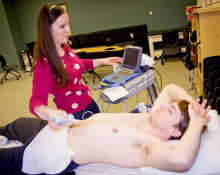
UK Medical Students Receive High Marks a Ultrasound Challenge
UK Medical Students Receive High Marks at Ultrasound Challenge
While the world watched coverage of the Winter Olympics last month, a group of University of Kentucky medical students were vying for medals in an Olympic-style competition for future doctors.
For the first time, a team of eight UK medical students participated in the 5th Annual Ultrasound Challenge at Ohio State University on Feb. 15. During the challenge, students from UK and The Ohio State University tested their knowledge, technique and accuracy scanning ultrasound images of specific systems in the human body.
Biobank Offers Way for Patients to Help Others
In addition to providing high quality health care, the University of Kentucky is also committed to finding new treatments, tests, and cures for diseases. Now, patients at UK can contribute to that effort by agreeing to participate in the Research Registry and Specimen Bank, or the "biobank."
When you have a medical procedure that involves, for example, drawing blood or sampling tissue, there is often material left over from the testing process that would otherwise be thrown away.
UK Conference to Focus on Health Disparities in Appalachia
Nearly 700 researchers, students, policymakers and community members will gather at the Lexington Convention Center March 27 to share research, mentor junior faculty, and enhance collaborations in clinical and translational science, with special focus on addressing health disparities in Appalachia.
Active Investigators to Update Financial Disclosure Forms in March
All active investigators will receive a reminder to update their Financial Disclosure Forms during the month of March. EAG is sending the email in batches. The first was sent on February 18 and they are currently through the S’s.
Current F&A Rates for Clinical Trials
As a reminder, clinical trial F&A rates for industry-funded studies increased effective 7/1/2013. Please be sure to use this rate in study budgets being discussed with sponsors. Information can be found here: http://www.research.uky.edu/ospa/info/clinical_info.html.
UK Pediatricians Publish Comprehensive Textbook about Newborn Kidney Disease
UK HealthCare pediatricians Dr. Aftab S. Chishti and Dr. Stefan G. Kiessling, have edited a new textbook that provides in-depth clinical instruction about the treatment of kidney and urinary tract diseases in newborns.
Published in January, "Kidney and Urinary Tract Disease in Newborns" provides doctors with comprehensive, practical knowledge for the diagnosis and treatment of kidney diseases in babies younger than a year old. The textbook includes contributions from more than 20 experts in the field of pediatric nephrology.